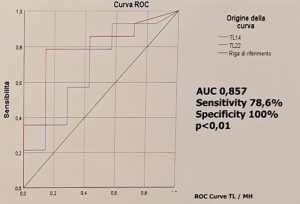
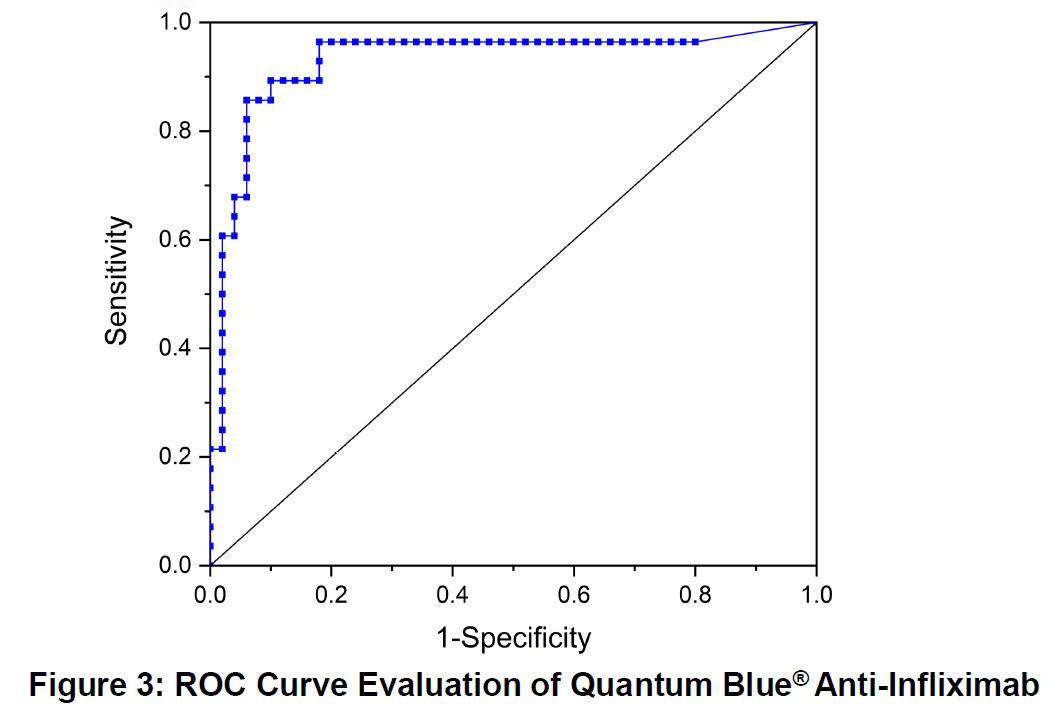
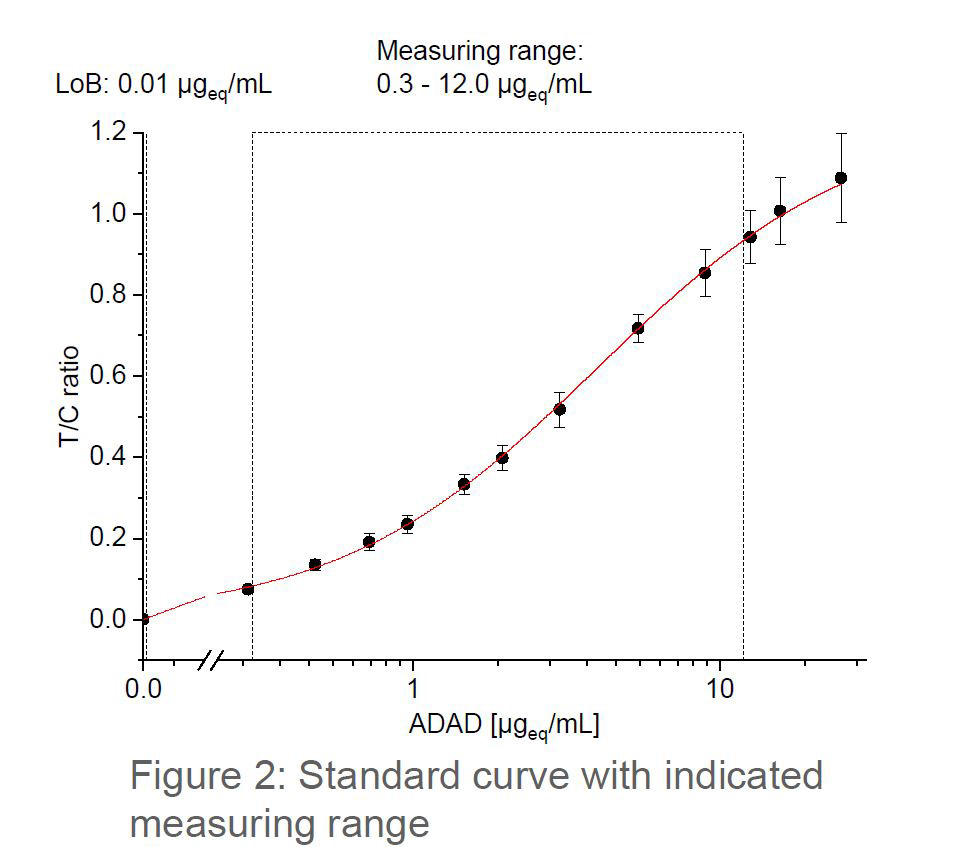
Serum trough levels for biologic drugs are extremely useful in determining effective treatment. These compounds are prone to either a primary loss of response (up to 30% of patients) or a loss of response over time (up to 46% of patients) with the level varying depending on the compound.
Historically, testing has been performed using ELISA methods which necessitates batch testing in order to be cost efficient but inevitably introduces a delay in obtaining the result. This is compounded by the fact that few hospitals run the analysis themselves – most use a referral service which means results can be delayed by several weeks.
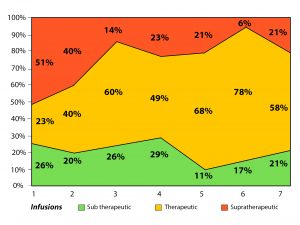
The optimal time to assess a drug level is immediately prior to the next infusion when it is at the trough level. Consequently, due to the time taken to obtain test results traditionally, patients will receive at least the immediate dose without adjustment. They may even receive multiple further doses before the drug can be optimised into the therapeutic window.
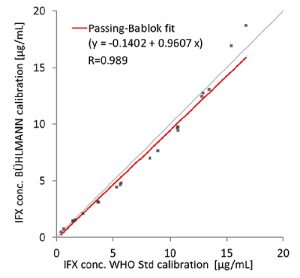
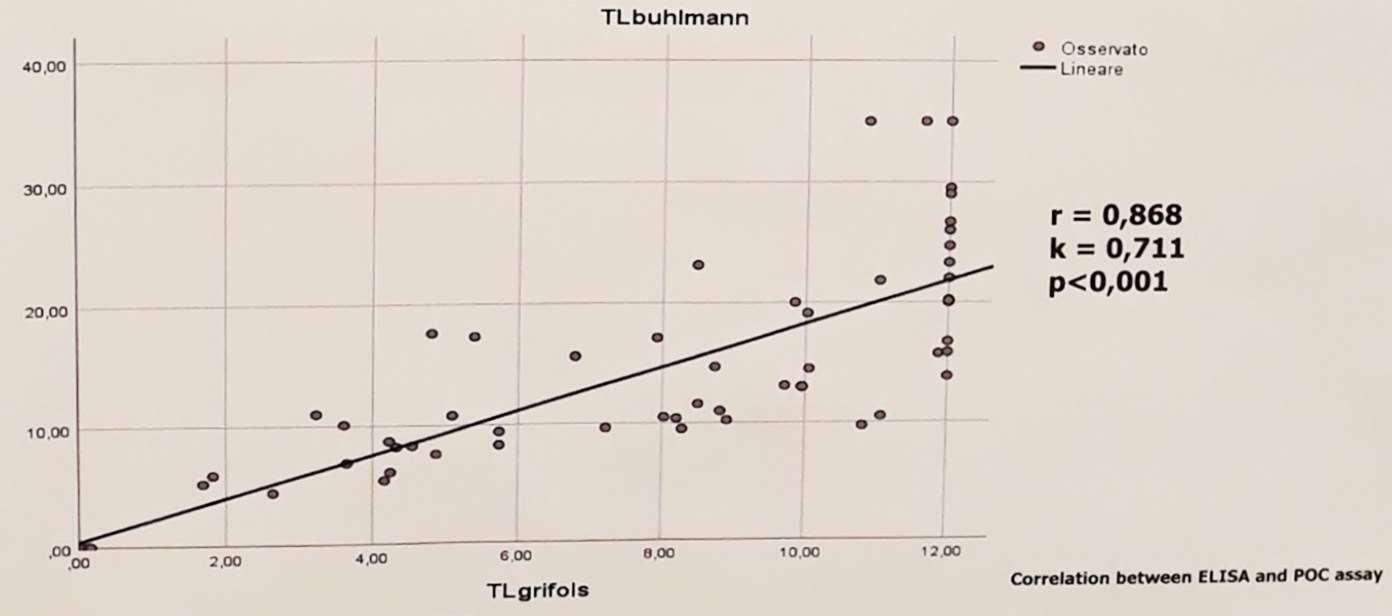
In a poster presented by C. Rentsch et al. at ECCO 2018, data showed 77% of patients required dose adjustment based on the serum trough levels achieved after the first dose, with 51% requiring dose reduction and 26% requiring dose escalation. (See graph on right)
In the absence of timely trough level information these patients are likely to receive subsequent doses without the appropriate adjustment, therefore compounding the situation.