Calprotectin is a soluble protein that is found in white cells; it is part of the bodies defence mechanism and has strong antibacterial and antifungal properties through its ability to sequester metal ions. Calprotectin is actually formed from two proteins, S100-A8 and S100-A9 (alternatively known as MRP-8 and MRP-9) and it is the heterodimer of these two proteins that is found inside the white cells. When calprotectin is released from white cells, it initiates a strong inflammatory response and rapidly forms a tetrameric format in conjunction with metal ions (calcium, zinc, magnesium).
There is as much calprotectin in white cells as there is haemoglobin in red cells accounting for around 60% of the soluble protein content. In conditions which provoke an inflammatory response the level of calprotectin in the blood can increase significantly and although it is a non-specific marker, it can be used to aide diagnosis and determine the severity of certain conditions in conjunction with the other clinical evidence. The diagnostic advantage of calprotectin detection over other disease markers is that it is stored in the cell and released immediately in response to local situations. In contrast, other markers may be released by downstream pathways or need to be synthesised leading to delays in detection.
Although calprotectin is a non-specific marker, there have been publications to show it to be of value in the diagnosis and monitoring of various conditions including:
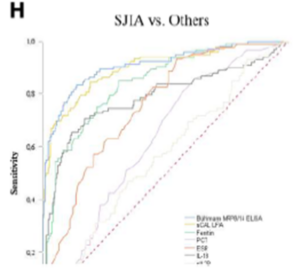
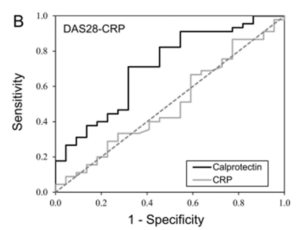
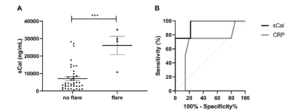
- Rheumatoid and juvenile idiopathic arthritis
- An early marker for sepsis
- An early marker for acute coronary syndromes
- Idiopathic pulmonary fibrosis
- Behcet’s disease
- Stills disease
- Systemic lupus erythematosus
- Systemic sclerosis
- Primary Sjorgens Syndrome