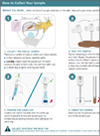
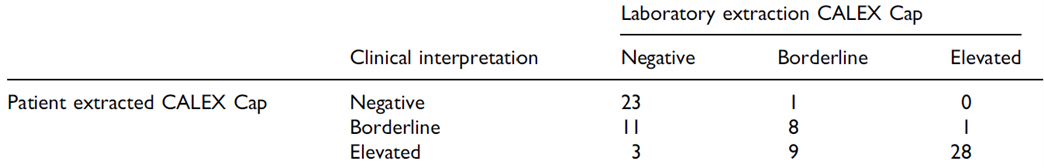
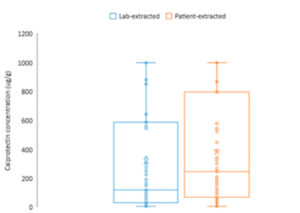
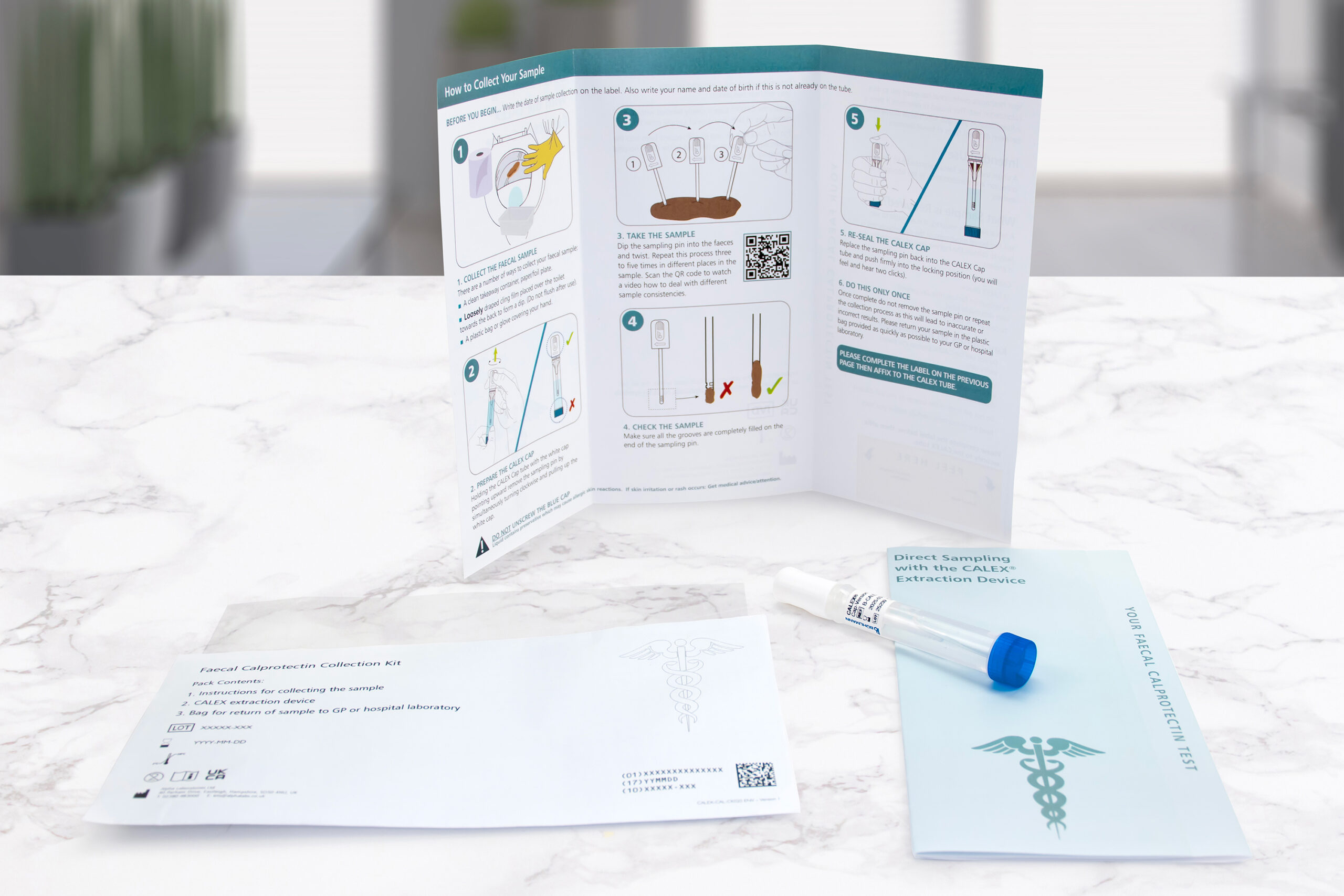
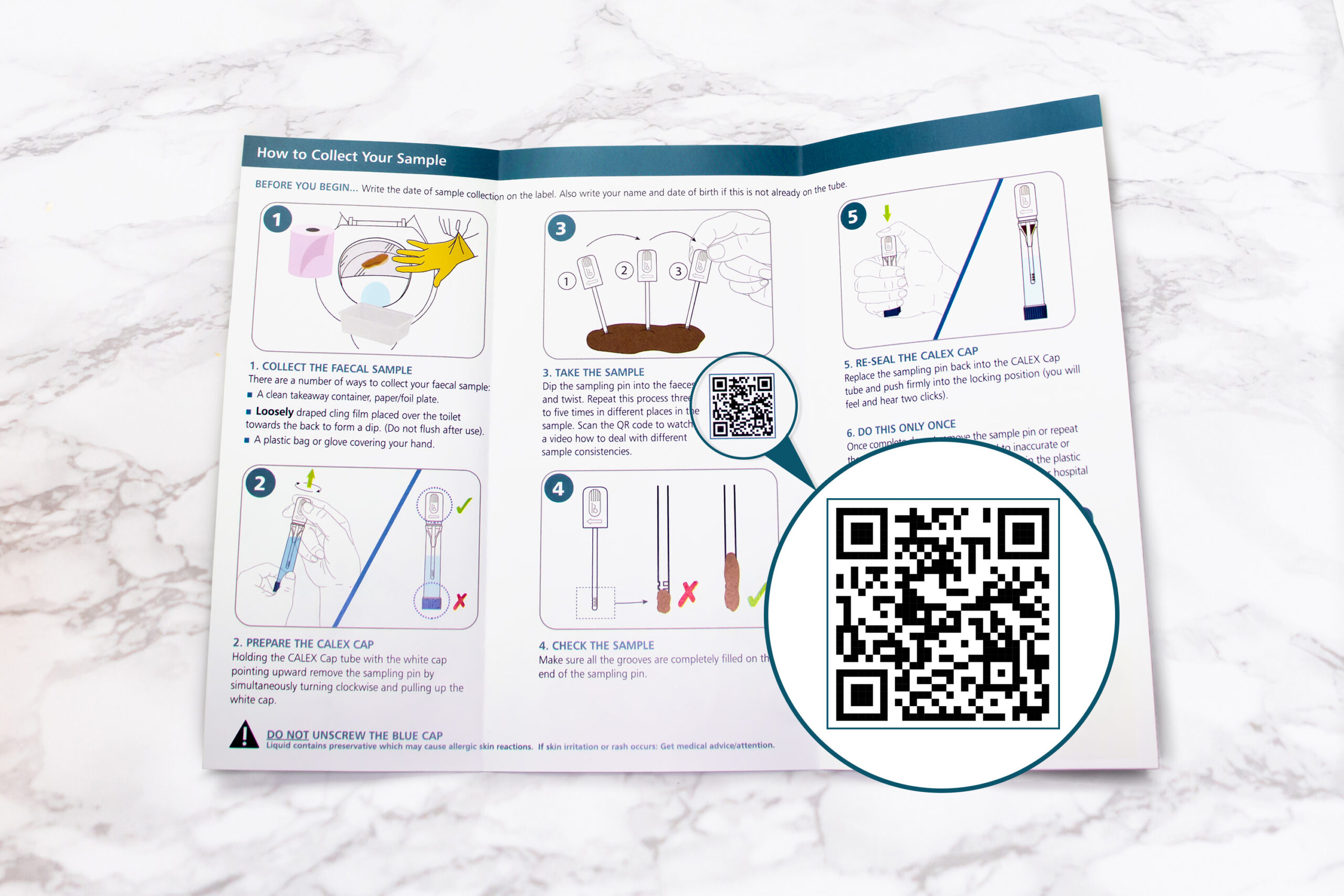
Introduction of the CALEX Cap in 2014 revolutionised the workflow for faecal calprotectin extraction within the laboratory with rapid, clean and consistent sample extraction.
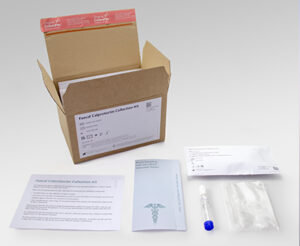
Now CALEX patient packs are available to enable the patient to prepare the CALEX and send to the laboratory rather than a stool sample in a pot which will transform the way calprotectin testing is performed.
Receiving the CALEX rather than a stool sample that requires extraction will release huge amounts of resource in the laboratory, transforming the workflow. On receipt the CALEX will be ready to vortex, centrifuge and load onto the analyser for testing.