In 2013 NICE published the DG11 guidance supporting the use of calprotectin as a cost-effective method to differentiate between IBD and IBS. Since that time, the level of testing within laboratories has increased significantly and BÜHLMANN has expanded its product portfolio to assist with evolving hospital requirements. BÜHLMANN offers the widest range of calprotectin assays, all standardised together to help support testing across the healthcare network.
With the implementation of NHS Improvement Pathology Networking, many trusts are now operating on a hub and spoke model with locations taking responsibility for specific tests within the trust. This can save costs, but potentially could also result in a reduced service in the spoke settings.
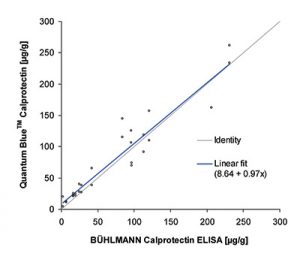
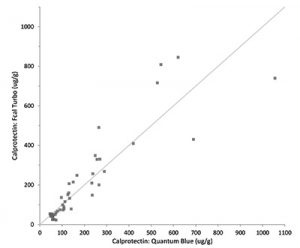
Placing a Quantum Blue reader in spoke settings enables for single tests to be performed when there a requirement for a rapid result to help support clinical decision making and giving a result that will correlate with the other BÜHLMANN assays, be that the fCAL® turbo, fCAL® ELISA or the IBDoc®.
It is simple to use in either the clinic or laboratory setting.
- Based on established lateral flow technology
- Quantitative measurement of Calprotectin in faecal samples
- Results in 12 minutes
- Excellent correlation with other BÜHLMANN Calprotectin assays
- Simple, rapid extraction and test process
- Unilateral connectivity to LIMs via dedicated middleware
- Compact benchtop reader with touch screen or optional barcode data entry
- Results directly printable or downloadable to USB
- There are two kits available for testing calprotectin in stool samples:
Quantum Blue® fCAL High Range (LF-CHR25) – measurable range 100-1800µg/g for monitoring IBD positive patients
Quantum Blue® fCAL Extended Range (LF-CALE25) – measurable range 30-1000µg/g for diagnosis and monitoring purposes