Stool analysis for biomarkers such as faecal calprotectin and elastase provides a non-invasive tool to investigate the condition of a patient’s digestive health and help diagnose disease, such as inflammatory bowel disease and pancreatic insufficiency respectively.
Woodley et al Torbay and South Devon NHS Trust- [email protected]
- +44 (0) 23 8048 3000
Calprotectin Extraction
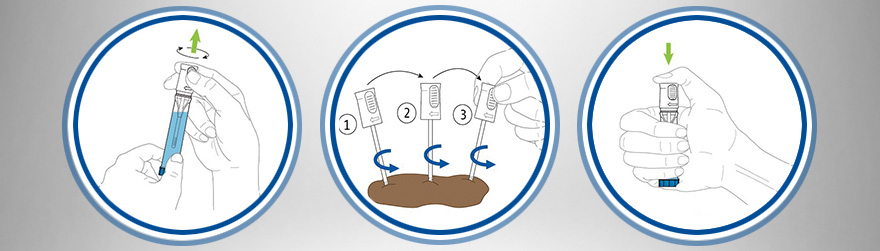
Poo-tentially better? Faecal calprotectin and elastase stability in different sample collection devices and a trial of at home patient-led extraction
Evaluating the service user experience of CALEX® cap devices as a faecal sample collection method for calprotectin testing: a local audit
Overall, the results affirm that CALEX® cap devices are easy-to-use with accessible instructions and are highly rated over universal tubes by patients. Some invaluable feedback was also obtained, enabling the creation of strategies to improve the service and ensure that faecal sample collection is easy, clear, and comfortable for NHSL service users.
Ralph et al UKMedLab 2023BÜHLMANN Calex® Cap for the faecal extraction of calprotectin – Fit for purpose?
Calex® devices are fit for purpose, easy to use and offer a quicker extraction process compared with manual weighing and will therefore enable increasing demands of a faecal calprotectin service.
Read the Letter to the Association for Clinical Biochemistry & Laboratory MedicineHow to Prepare an Extraction Control
Preparation of a simple internal quality control for Faecal Calprotectin, from Jane French, Consultant Clinical Scientist, UK NEQAS.
“As Programme Director of the UK NEQAS for Faecal Markers, I am often asked if I can recommend a suitable internal quality control (IQC) material for Faecal Calprotectin. Until quite recently, there has been no commercially-produced IQC available but all is not lost – with a bit of know-how, it is perfectly possible to make your own bespoke IQC, tailored to your exact requirements.”
Read ‘Everything is Under Control!’ from Leading Edge – 2019 Issue 2Fecal Calprotectin Quantification Demands For Enhanced Automation
The manual weighing method with a minimal amount of 50-100 μg fecal sample extracted in 50 volumes of extraction buffer was established in the early 2000 and guarantees the best possible quantification of fCAL. The method is regarded as the reference method for fCAL quantification. New devices simplifying the time consuming reference method have been introduced by many different immunoassay manufacturers. The new CALEX® Cap extraction device from BÜHLMANN was validated in this study.
Niederberger, C. et al. (2018) Nordic Congress in Clinical ChemistryEvaluation of BÜHLMANN CALEX® Cap Stool Extraction Devices for the extraction of faecal calprotectin
Calex® extraction devices compare well to extraction via manual weighing. Calex® also demonstrated similar imprecision and accuracy to manual extraction.
The main benefits of Calex®, speed and ease of use, will enable higher throughput and will allow the service to cope with future increases in demand.
The devices are also more hygienic and the significant reduction in staff time and other reagents/consumables will no doubt result in an overall cost saving.
CALEX® Cap, A novel stool collection and extraction device:Performance Analysis and Stability of Calprotectin in Stool Extracts
The stool sampling using CALEX® Cap is very reproducible, easier and more hygienic than manual weighing. The extraction of
calprotectin from stool samples is as reliable as with conventional methods. CALEX® Cap represents a fully quantitative “all-in-one” device.
Same faeces, same lab, different methods and some different calprotectin results
Overall the Eurospital extraction devices had more disadvantages than the BÜHLMANN Calex tubes, being more laborious with an acceptable risk of splashing the extract
Lythgoe and McCann;Stockport NHS – Poster BSG 2016Evaluation of the BÜHLMANN fCAL Turbo Faecal Calprotectin Assay on the Abbott Architect C8000. Comparison to the Phadia EliA and the IBDoc® home test kit
fCAL turbo is very adaptable to the Abbott Architect C8000 analyser. The CALEX ® cap extraction devices are easy to use and practical. Inter-assay variability demonstrated, and recommends users to participate in the UK NEQAS Scheme . No interference to any of the other chemistries.
O’Connell (2017) Research Project Scientific Paper